Abstract
Background: Results of a phase I trial of donor-derived CD7 chimeric antigen receptor (CAR) T cells for relapsed or refractory T-cell acute lymphoblastic leukemia (r/r T-ALL) (Pan et al. J ClinOncol 2021;39:3340-3351) have been reported previously. Herein, we report interim findings of the phase 2 trial on the efficacy and safety of donor-derived CD7 CAR T cells for relapsed or refractory T-cell acute lymphoblastic leukemia/lymphoma.
Methods: The subjects in phase 2 were screened from the similar I/E criteria of phase 1 trial. Patients with prior stem cell transplantation (SCT) received CAR T cells from prior SCT donors, while patients without SCT history received CAR T cells from new donors who also provided stem cells for transplantation post CAR T therapy. The phase 2 trial was using Bayesian optimal phase 2 (BOP2) design to evaluate the efficacy and safety of the target dose of 1 × 106 (±20%) CD7 CAR T cells per kg of body weight in refractory/relapsed T-cell acute lymphoblastic leukemia/lymphoma (NCT04689659). The primary endpoint was efficacy with safety secondary. An interim analysis of phase 2 trial could be performed when the first 20 patients who receive CD7 CAR T cells have completed 3 months from infusion or discontinued earlier. Survival status were continuously followed up while severe adverse events (SAEs) were recorded until receiving other anti-leukemia therapy.
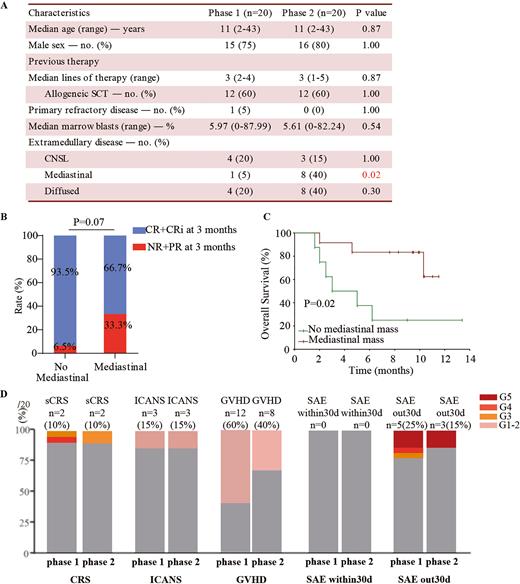
Results: Eighteen (90%) of twenty enrolled patients responded in phase 2 trial and were followed up with a median time of 11.0 months (range 7.6-14.6) until May, 14th, 2022. Patients in phase 1 and 2 trial had similar baseline characteristics, whereas phase 2 trial included more patients with mediastinal malignancies.(Fig. A)
The best overall response (BOR) rate was 90% at 3 months. The objective response rate (ORR) were 90% and 90% at 1 and 3 months post-infusion, which were similar to 95% and 95% at 1 and 3 months in phase 1 trial. The 3-months complete remission (CR) rate in patients with mediastinal tumors was lower than that in patients without mediastinal tumors, although there was no significant statistical difference (p=0.07, Fig. B) Of total 18 responders that were followed up, 12 (67%) patients who received no further therapy, all had continuously detectable CAR T cells until the last visit, three remained in remission, seven had a relapse (three CD7+, and three CD7-, one unknown), and two died of infection; 8 (44%) patients proceeded to SCT and no CAR T cells were detectable after SCT, and among them five remained in remission, two had a relapse (CD7-), and one died of infection. Patients relapsed at a median time of 4 (range 1.6-10.5) months. The one-year progressive-free survival (PFS) and overall survival (OS) rates were 62.3% (95% CI, 38.3-86.3) and 60.0% (95% CI, 38.5-81.5). Patients without mediastinal mass had longer OS than patients with mediastinal mass. (p<0.05, Fig. C) Updated phase 1 data at a median 1.5-year follow-up, shows PFS and OS rates of 60.0% (95%CI, 38.4-81.6) and 70.0% (95%CI, 50.0-90.0)
Short-term adverse events included grade 3 or higher cytokine release syndrome (10%) and grade 1-2 graft-versus-host disease (GVHD, 40%), which were all reversible. Three late-onset (> 30 days post-infusion) SAEs occurred in 3 responders, including 1 grade 5 sepsis at month 2, 1 grade 5 fulminant hepatitis at month 2, and 1 grade 5 pneumonia at month 5. Severe infections occurred in 2/3 patients with no further therapy, and the total T cells in them reached a median count of 654.34/μL (range 41.87-1210.46), which were substantially lower than normal levels despite steadily increasing. The safety analysis was similar to the phase 1 trial of donor-derived CD7 CAR T cells. (Fig. D)
Conclusions: Interim results from a phase 2 trial of donor-derived CD7 CAR T cell therapy showed similar encouraging activity in treating r/r T-ALL with phase 1 trial. Relapse emerges as major issues impeding long-term outcomes. CD7-negative relapse was commonly observed under CAR T cell surveillance. Late onset GVHD and infections may occur and should be carefully managed. Patients with mediastinal mass had poor overall survival and needed been further investigated.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal